Alternatively missense mutations in the rod domain of the lamin a c gene may alter interactions with cytoplasmic proteins in particular intermediate filament components of the sarcomere the.
Central rod domain lamin.
An escherichia coli expression system was used to produce full length lamin a and lamin c and truncated lamins retaining the central alpha helical rod domain residues 34 388 but lacking various amounts of the amino terminal head and carboxy terminal tail domains.
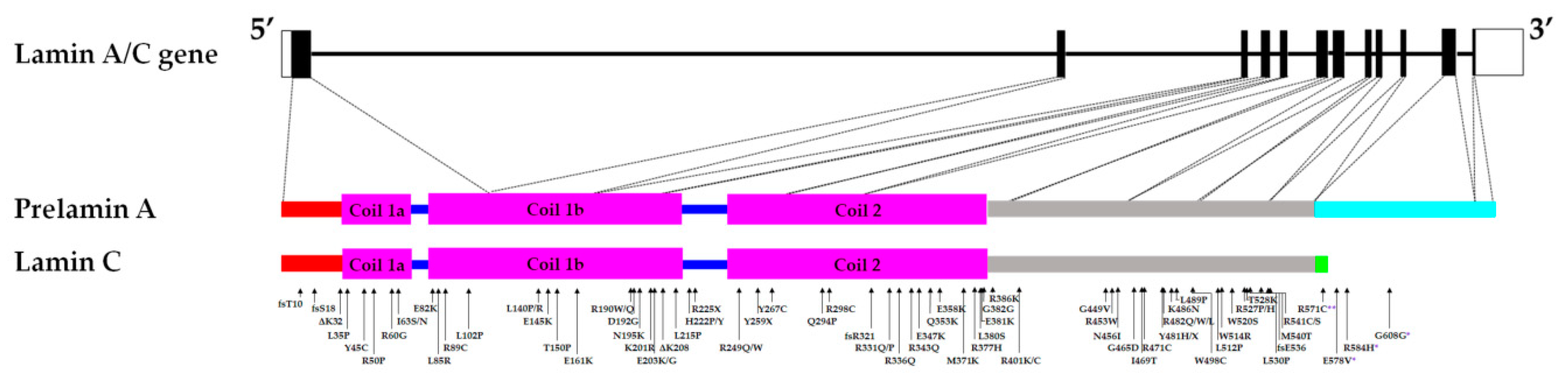
Arg190trp affects a conserved amino acid position supplementary data in the central rod domain of lamin a c which interacts with emerin and lamin b.
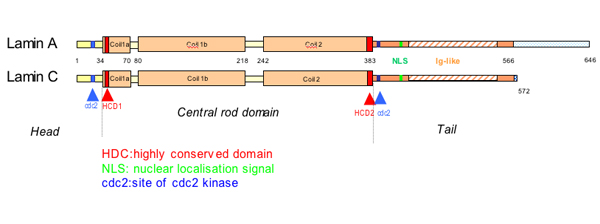
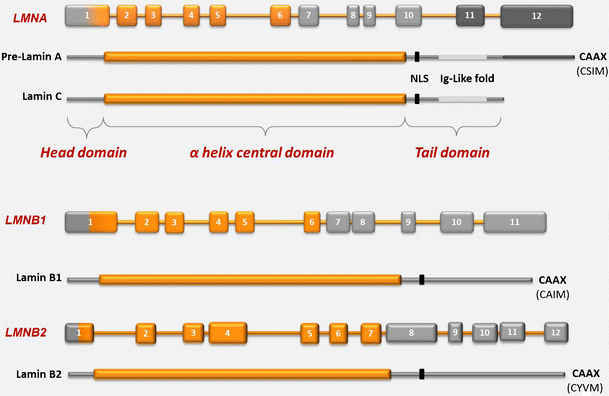
The lamins have a conserved central helical rod domain flanked by globular head and tail domains.
As suggested by the first model all if proteins appear to have a central alpha helical rod domain that is composed of four alpha helical segments named as 1a 1b 2a and 2b separated by three linker regions.
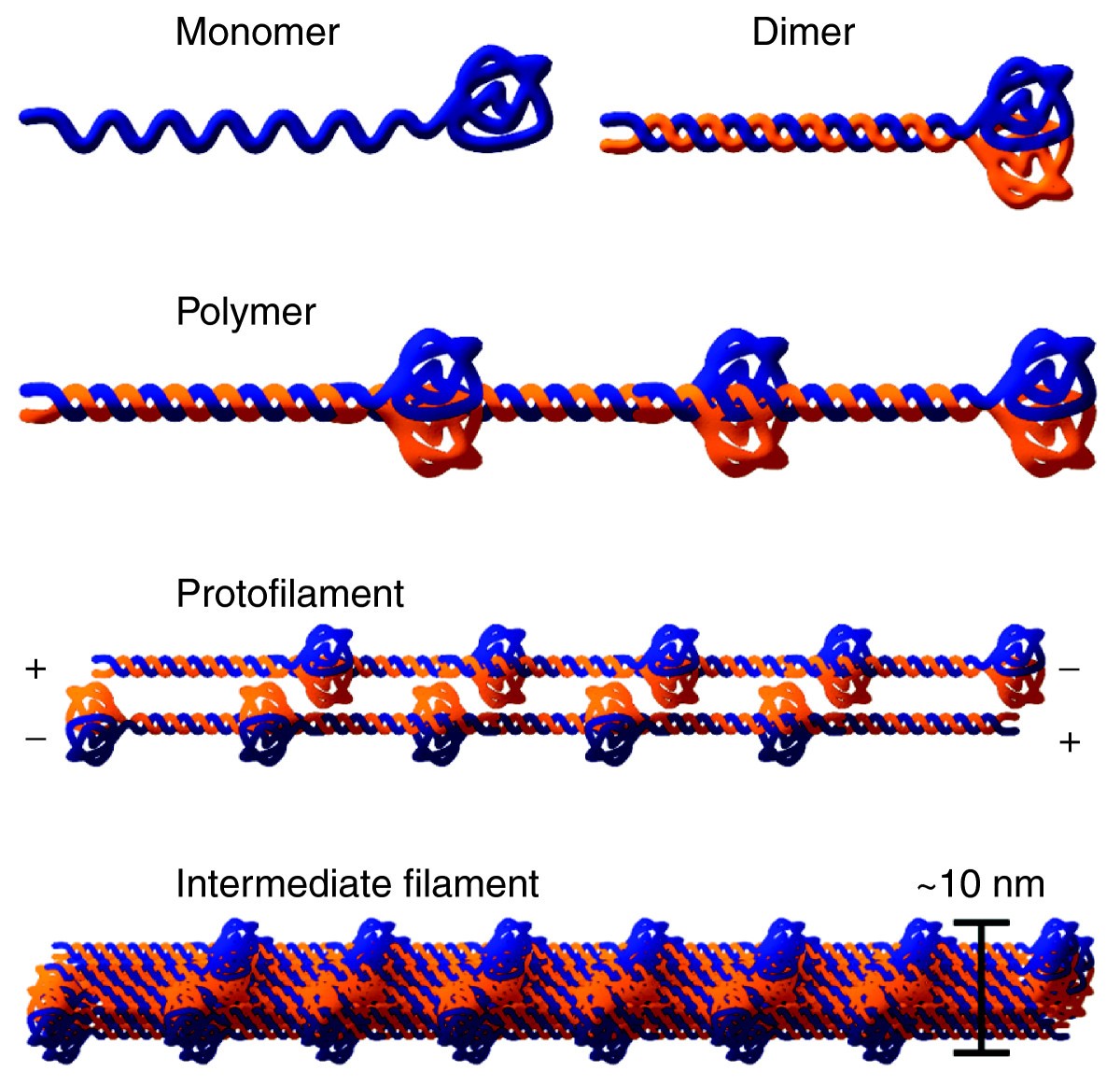
The central building block of an intermediate filament is a pair of two intertwined proteins that is called a coiled coil structure.
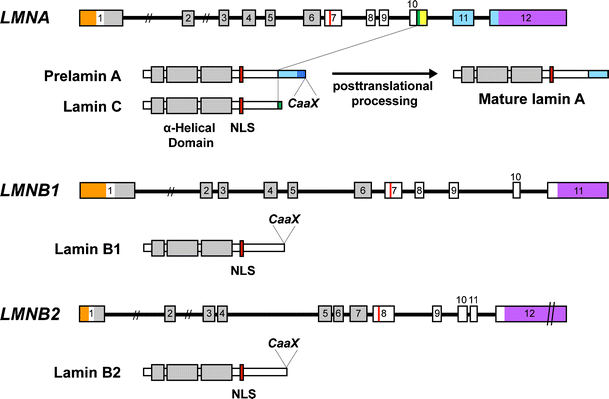
Intron positions are conserved in other lamin genes from frogs mice and humans but different in lamin genes from drosophila and c.
Exon 1 codes for the n terminal head domain and the first portion of the central rod domain exons 2 through 6 the central rod domain and exons 7 through 11 the c terminal tail domain.
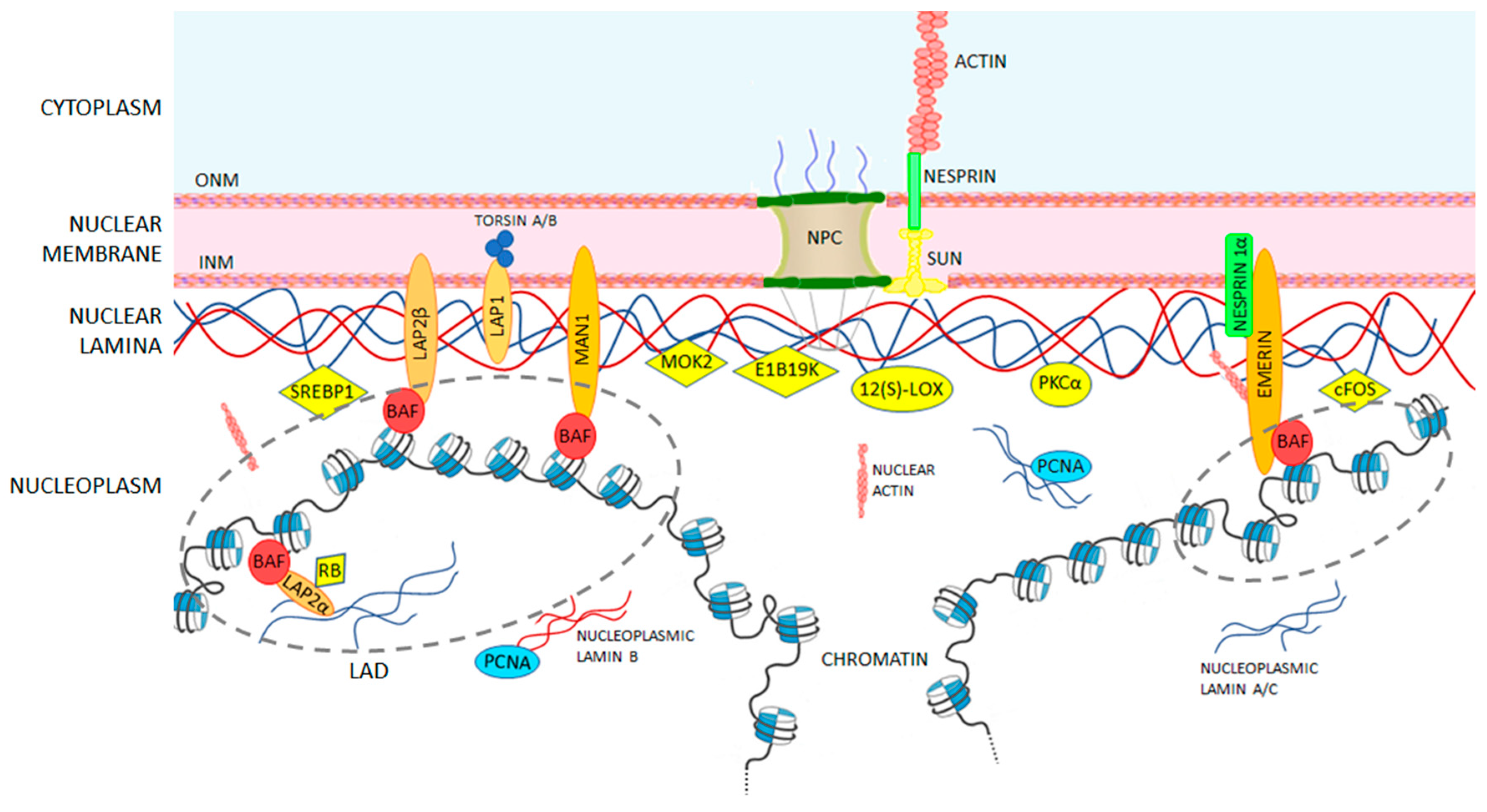
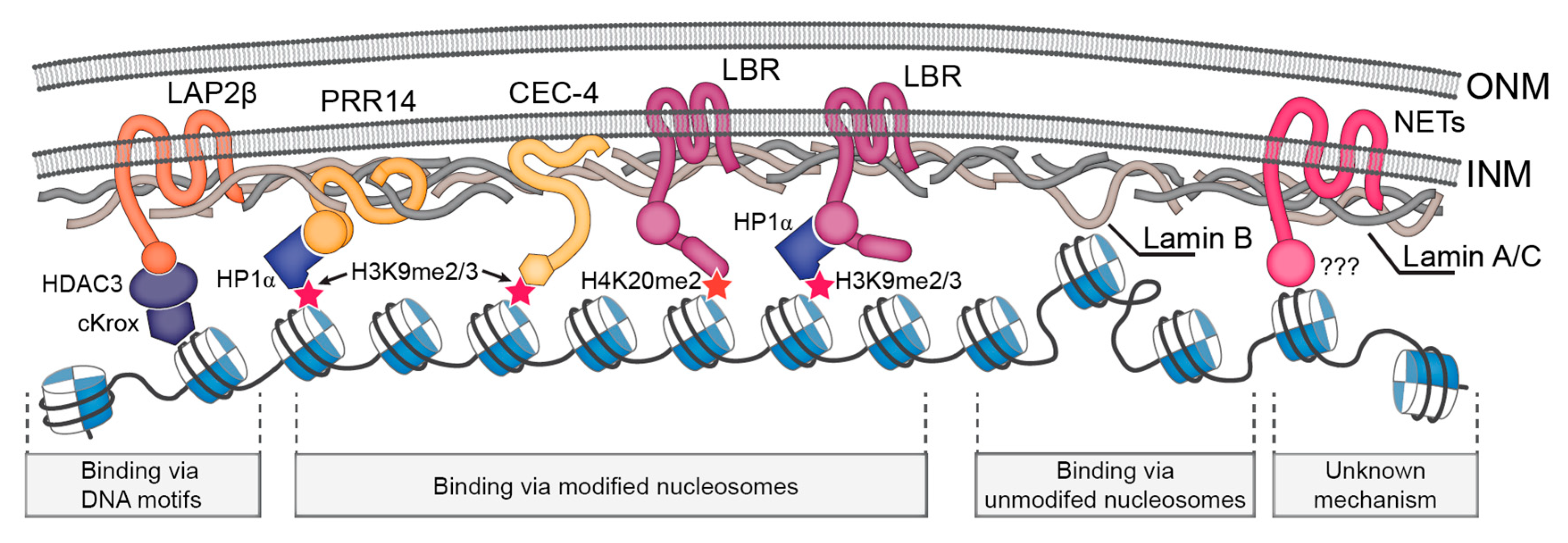
Assembly of the nuclear lamina occurs via polymerization involving homodimerization head to tail assembly of homodimers and antiparallel assembly of.
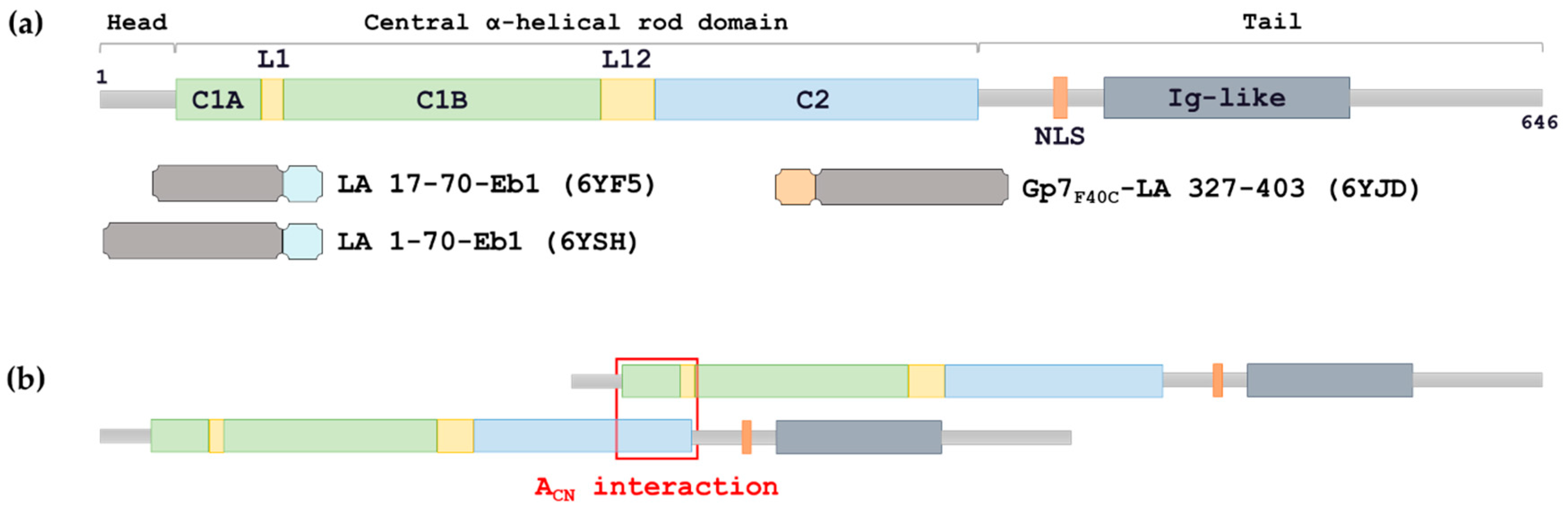
Lamins consist of an n terminal head domain a coiled coil central rod domain and a globular c terminal tail domain containing an immunoglobulin ig like fold which in vitro binds nucleosomes.
The central α helical or rod domain spans approximately half of the lamin molecule about 350 residues and comprises four α helical segments termed 1a 1b 2a and 2b.
An escherichia coli expression system was used to produce full length lamin a and lamin c and truncated lamins retaining the central alpha helical rod domain residues 34 388 but lacking various amounts of the amino terminal head and carboxy terminal tail domains.
Numerous mutations in the human a type lamin gene lmna cause the premature aging disease progeria.
The central rod is responsible for the formation of in parallel and in register coiled coil dimers the building blocks of lamin polymers.
Some of these are located in the α helical central rod domain required for the polymerization of the nuclear lamins into higher order structures.
24 it is reported in patients with dilated cardiomyopathy with atrioventricular block 25 and more recently in a family with dilated cardiomyopathy and lv non compaction.
In vitro lamin dimers form head to tail chains which further.