Follow the correct techniques and ask the instructor for help.
Chemistry of copper report sheet.
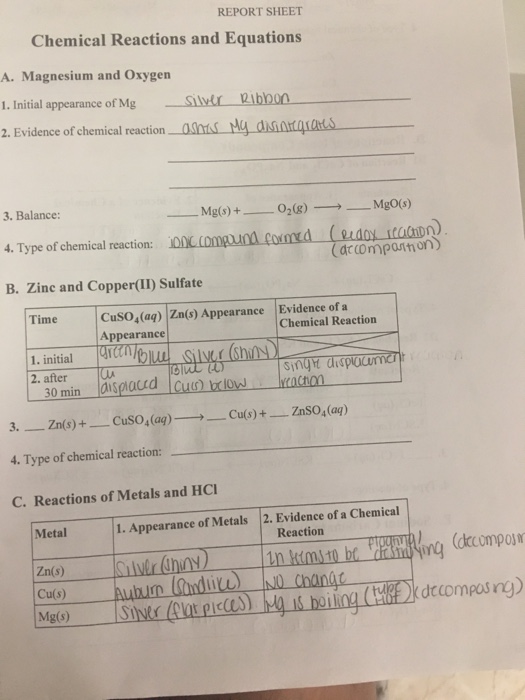
Cuso 4 aq zn s znso 4 aq cu s e quation example using your data m oles of hydrated copper ii sulfate.
Abstract the objectives of this experiment were to recover the 0 2 g cu from the beginning of the experiment and to classify the types of chemical reactions that took place.
Data sheet attached observations a.
Keywords separation sodium hydroxide copper erlenmeyer flask hydrogen chloride.
Copper i chemistry is limited by a reaction which occurs involving simple copper i ions in solution.
Copper i ions in solution disproportionate to give copper ii ions and a precipitate of copper.
Chemistry lab report copper cycle this is a lab report for my general chemistry class.
Chemistry of copper lab 3 pages 109 115 pre lab pages 111 112 post lab questions page 114 115.
Cuso ag n of the reactions that occured.
The chemistry of copper.
By reacting copper with several chemicals we were able to observe the various chemical changes that the copper underwent.
Locker 7c aug.
This particular lab report shows my ability to work with quantitative data and analyze the calculations and measurements from the lab.
The assignment was to create a formal lab report that expresses data and observations lab procedure and a discussion of the data with a conclusion.
0 like 0 tweet.
Data for copper cycle 1.
This is a good example of disproportionation a reaction in which something oxidises and reduces itself.
Mass of test tube g 2 mass of test tube g copper g 3.
Chemical reactions of copper and percent yield key objective to gain familiarity with basic laboratory procedures some chemistry of a typical transition element and the concept of percent yield.
Cu no3 2 aq red.
Essay by locoho college undergraduate a october 2006.
E2 failure to dry copper completely add to much water to the test tube.
Ap chemistry lab report.
Experiment 28 report sheet chemistry of copper lab sec name desk no.
Mass of copper sample g trial i trial 2 trial 3 instructor approvalbalanced equation observation synthesis of b cu oh 2 s oc.
18 copper wire evaporating dish 250 ml beaker 2 weighing paper concentrated hno.
The lab was to include a purpose procedure data observations all reactions and.
Apparatus and chemicals 0 5 g piece of no.
A 0 0194 g sample of copper metal is recycled through the series of reactions in this experiment.
Introduction copper is found in group 11 mw 63 456 shiny orange red color malleable ductile oxidizes in air turns a green color patina.
Mass of copper ii sulfate used 5 003g mass of zinc used 1 355g mass filter paper 225g mass of filter paper and copper 1 571g calculations.
Download word file 2 pages 4 3.